Introduction
Recent scientific research has brought to light the potential risks of using acetaminophen, known as Tylenol or Paracetamol, during pregnancy. Studies have indicated a correlation between acetaminophen use by expectant mothers and a heightened chance—ranging from 20% to 30%—of their children receiving a diagnosis of autism spectrum disorder (ASD), ADHD, hyperactivity, or other related conditions. These findings are derived from a series of large-scale studies characterized by consistent methodologies and robust designs, underscoring the reliability of the evidence presented.
Visiting scholar Parker, with a background in psychology and neuroscience and over 140 peer-reviewed articles to his credit, stresses the increased vulnerability of newborns to pharmaceuticals following the severance of the umbilical cord, which removes the protective filter of the mother's liver. This perspective aligns with the ongoing product liability lawsuits against Tylenol's manufacturers, who stand accused of inadequately warning about these potential risks.
While autism spectrum disorder can manifest due to a combination of genetic and environmental factors, the role of environmental influences—such as prenatal exposure to certain medications—is an area of growing concern, especially given the prevalence of ASD, which affects approximately 1 in every 50 to 60 children.
The gravity of these findings has led to numerous product liability lawsuits, with the legal proceedings rigorously evaluating the scientific data on prenatal acetaminophen exposure. As a result, individuals affected are now seeking to understand their eligibility for a potential Tylenol-autism claim settlement, a reflection of the significant impact this issue has on families and the wider public.
Background of Tylenol Autism Lawsuits
The intersection of Tylenol use during pregnancy and the risk of autism spectrum disorders (ASD) has ignited a series of product liability lawsuits. Major pharmaceutical companies are currently facing allegations that they failed to adequately warn the public about the dangers associated with prenatal acetaminophen exposure. Research indicates a 20% to 30% heightened risk of ASD, ADHD, and other related disorders in children when acetaminophen is used during pregnancy.
With robust studies backing these claims, the legal landscape is teeming with approximately 500 lawsuits under the scrutiny of Judge Cote, and thousands more are being examined by legal experts. The legal proceedings have intensified discovery efforts into the Tylenol-autism connection, while also addressing challenges to expert witness credibility. Meanwhile, settlement discussions are underway, offering a potential resolution for families affected.
The gravity of this issue is underscored by the fact that acetaminophen is present in many common over-the-counter remedies, raising concerns for the millions of users worldwide.
Allegations and Claims in Tylenol Lawsuits
The connection between Tylenol usage during pregnancy and the onset of autism spectrum disorder (ASD) in children has become a focal point of numerous legal cases. Plaintiffs in these lawsuits assert that manufacturers and sellers of Tylenol and its generic counterpart, acetaminophen, did not adequately warn expectant mothers, healthcare professionals, or the public about the potential risks linked to the drug's use during pregnancy. The allegations are grounded in research suggesting a 20% to 30% increase in the diagnosis of ASD, ADHD, and other behavioral disorders in children following prenatal exposure to acetaminophen.
These legal actions, part of a broader category known as mass tort lawsuits, leverage large-scale studies with consistent methodologies and robust design to support claims that acetaminophen use during pregnancy may significantly heighten the risk of neurodevelopmental issues in children. The scientific community, including experts like visiting scholar Parker from the University of North Carolina, Chapel Hill, has weighed in on the matter. Parker emphasizes the vulnerability of newborns to pharmaceuticals immediately after birth, as they no longer have the protection of the mother's liver once the umbilical cord is severed.
The scrutiny of Tylenol's potential risks has not been without controversy. The drug is widely used, with more than 50 million American adults taking it weekly, and is considered safe for adult consumption. However, the debate intensifies when considering the delicate developmental stages of a fetus and a newborn.
The litigation has prompted calls for clearer labeling, akin to warnings used in Europe, which caution about the potential increase in autism and ADHD risks with frequent use during pregnancy.
Recent research led by Woodbury and Schantz at the Beckman Institute for Advanced Science and Technology has furthered the discussion by linking higher acetaminophen exposure during pregnancy to language delays in children, a key indicator of future cognitive abilities. These findings, coupled with the recommendations of the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine to use acetaminophen as the first-line treatment for pain and fever during pregnancy, highlight a complex landscape where the need for pain relief must be balanced with the emerging evidence of potential risks to child development.
Scientific Studies on the Link Between Tylenol and Autism
Recent scientific research has brought to light the potential risks of using acetaminophen, known as Tylenol or Paracetamol, during pregnancy. Studies have indicated a correlation between acetaminophen use by expectant mothers and a heightened chance—ranging from 20% to 30%—of their children receiving a diagnosis of autism spectrum disorder (ASD), ADHD, hyperactivity, or other related conditions. These findings are derived from a series of large-scale studies characterized by consistent methodologies and robust designs, underscoring the reliability of the evidence presented.
Visiting scholar Parker, with a background in psychology and neuroscience and over 140 peer-reviewed articles to his credit, stresses the increased vulnerability of newborns to pharmaceuticals following the severance of the umbilical cord, which removes the protective filter of the mother's liver. This perspective aligns with the ongoing product liability lawsuits against Tylenol's manufacturers, who stand accused of inadequately warning about these potential risks.
While autism spectrum disorder can manifest due to a combination of genetic and environmental factors, the role of environmental influences—such as prenatal exposure to certain medications—is an area of growing concern, especially given the prevalence of ASD, which affects approximately 1 in every 50 to 60 children.
The gravity of these findings has led to numerous product liability lawsuits, with the legal proceedings rigorously evaluating the scientific data on prenatal acetaminophen exposure. As a result, individuals affected are now seeking to understand their eligibility for a potential Tylenol-autism claim settlement, a reflection of the significant impact this issue has on families and the wider public.
Recent Findings: No Causal Link Established
While discussions surrounding the use of Tylenol (acetaminophen) during pregnancy continue, recent scientific investigations have provided more nuanced insights into its possible effects. Lawsuits allege that manufacturers did not adequately inform the public about the risks associated with Tylenol use during pregnancy, which some studies have linked to a 20% to 30% increased likelihood of autism spectrum disorder (ASD) and other neurodevelopmental disorders in children. These concerns are backed by research that points to robust study designs and consistent methodologies underscoring a possible connection between prenatal acetaminophen exposure and subsequent diagnoses in children.
Autism, a disorder that manifests in early childhood and affects social interactions and behavior, has been studied for its potential links with various environmental factors, including medication exposure during pregnancy. In light of this, research has been conducted to examine whether acetaminophen, a common pain reliever recommended as the first-line option for pregnant individuals, could be one such factor influencing child health outcomes. Studies vary, with some reporting no significant associations, while others, especially larger and more frequent assessments of acetaminophen use during pregnancy, indicate potential correlations with motor and attention delays, as well as behavioral issues in children.
It's noteworthy that while the FDA underscores the safety of many medications, including the lack of a link between vaccines and autism, the question of acetaminophen's impact during pregnancy remains under examination. With 50–65% of pregnant individuals in North America and Europe reporting at least one instance of acetaminophen use, the drug's prevalence makes understanding its safety profile all the more critical. Researchers like Parker, with extensive experience in studying the developing brain, suggest that while the fetus has some protection during pregnancy, neonates are not as equipped to process pharmaceuticals, highlighting the importance of timing and exposure levels.
As legal cases unfold and the scientific community continues to explore this issue, parents and healthcare providers alike are encouraged to stay informed about the latest research findings to make well-considered decisions regarding medication use during pregnancy.
Public Health Implications and Precautionary Measures
Amidst the growing concern over the safety of medications during pregnancy, acetaminophen, widely known as Tylenol, has been scrutinized for its potential link to developmental disorders in children. Recent product liability lawsuits allege that manufacturers and retailers did not adequately warn the public about the risks associated with acetaminophen use during pregnancy. Studies have suggested a 20% to 30% increased risk of children being diagnosed with autism spectrum disorder (ASD) and other neurodevelopmental issues when exposed to the drug in utero.
These revelations are prompting healthcare professionals to reevaluate their recommendations for medication during pregnancy. Dr. Parker, a distinguished researcher with over 140 peer-reviewed articles to his name, highlights a critical transition point at birth when the protective role of the mother's liver is no longer present, making newborns particularly vulnerable to pharmaceuticals.
Moreover, ongoing investigations have produced robust evidence indicating a significant correlation between prenatal acetaminophen exposure and the diagnosis of neurodevelopmental disorders. This evidence is shaping public health dialogues and legal actions, with the potential to influence future healthcare policies and practices.
For parents and healthcare providers, it is increasingly important to consider these findings when making decisions about medication use. Conversations about drug ingredients and potential interactions with other medicines or supplements are essential. Transparency about the components of over-the-counter products, including the presence of acetaminophen, is vital for informed decision-making.
The implications of these developments are far-reaching, highlighting a need for caution, deeper understanding, and a proactive approach to ensuring the well-being of children in the face of potential medication-related risks.
FDA Involvement and Regulatory Responses
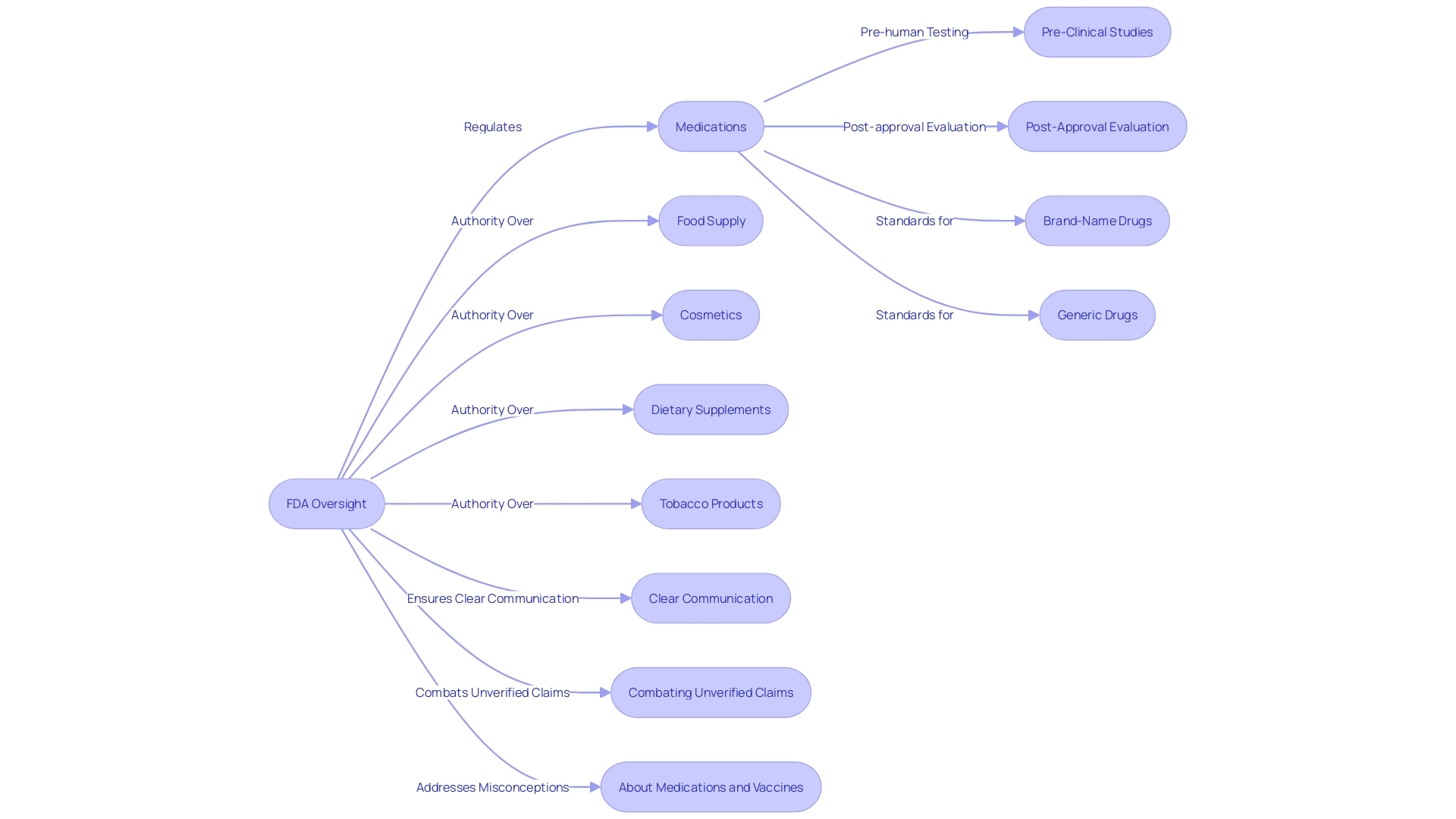
The U.S. Food and Drug Administration (FDA) plays a pivotal role in overseeing the safety and effectiveness of medications, an obligation extending to its response to concerns regarding Tylenol and autism. The FDA's comprehensive approach to drug regulation involves a continuous lifecycle assessment—from pre-human testing studies to continuous evaluation post-approval. With the recent publication of the "Direct-to-Consumer Prescription Drug Advertisements: Presentation of the Major Statement in Television and Radio Format Final Rule," the FDA underscores its commitment to clear communication.
This rule mandates that drug advertisements present major side effects and contraindications in an understandable manner, with concurrent audio and text for TV ads.
Notably, the FDA's rigorous standards apply uniformly to both brand-name drugs and FDA-approved generics, ensuring equivalent safety, efficacy, and quality. The agency's authority also extends to the nation's food supply, cosmetics, dietary supplements, and tobacco products, further emphasizing public health protection. Amidst the dissemination of erroneous online information about medical products, the FDA's role becomes even more critical.
Unverified claims can lead to unsafe and ineffective treatment choices, which the FDA actively combats through regulatory compliance and the dissemination of reliable information.
In the pharmaceutical industry, FDA regulatory compliance is a complex affair, with amendments to regulations potentially affecting the cost and timeline of drug development. The FDA's approval process, managed by the Center for Drug Evaluation and Research (CDER), is a thorough and lengthy endeavor, typically spanning 12 to 15 years. It involves an independent evaluation of a drug's safety and efficacy by expert physicians and scientists.
Furthermore, the 21st Century Cures Act of 2017 has adjusted the standards for drug approval, allowing for greater flexibility in the evidence required.
As part of its regulatory activities, the FDA also addresses misconceptions about medications and vaccines, evidenced by a multitude of studies debunking the supposed link between vaccines and autism. Such efforts highlight the FDA's critical role in both safeguarding public health and guiding informed medical decisions, reflecting the agency's steadfast commitment to consumer safety.
Impact on the Pharmaceutical Industry
The implications of the alleged link between Tylenol, a common acetaminophen-based medication, and autism are far-reaching for the pharmaceutical industry. Amid growing legal battles, companies face product liability lawsuits alleging failure to warn consumers, particularly pregnant women, about the potential risks associated with acetaminophen use. Recent studies have shed light on these risks, citing a 20% to 30% increase in the diagnosis of autism spectrum disorder (ASD) and other neurodevelopmental disorders in children following prenatal exposure to the drug.
This has not only impacted the marketing and sales strategies of these companies but also raised questions about their reputations. Despite a notable legal victory that quelled immediate concerns about financial settlements, the volume and consistency of research on this matter have led to a surge in litigation. With around 500 active lawsuits and thousands more under investigation, the potential for a significant increase in claims looms over the industry.
Moreover, the court's emphasis on further investigation into Tylenol's link to neurodevelopmental disorders, coupled with the ongoing settlement negotiations, underscores the seriousness of the issue at hand and the public's demand for accountability.
Current Legal Status and Ongoing Litigation
As the complex legal proceedings continue in the case of Barton et al. v. Reckitt Benckiser LLC et al., stakeholders are closely observing the unfolding events in the United States District Court of New Jersey (Case No. 2:23-cv-20370).
These lawsuits are a significant development in the accountability of pharmaceutical companies, alleging that the makers and retailers of Tylenol and generic acetaminophen products failed to adequately warn of risks when used during pregnancy. Specifically, they are associated with an increased incidence of autism spectrum disorder (ASD) and other neurodevelopmental disorders in children by 20% to 30%. Legal experts, including seasoned writer Sarah Edwards, adept at clarifying intricate legal matters, are weighing in on the case's intricacies and potential outcomes.
With a robust body of research supporting the plaintiffs' claims, the resolution of these cases could have far-reaching implications for product liability and consumer safety. Attorneys from firms such as DiCello Levitt are at the forefront, advocating for justice and offering insights into the litigation process through available media interviews.
Conclusion
Recent scientific research has highlighted the potential risks of using acetaminophen (Tylenol or Paracetamol) during pregnancy. Studies have shown a correlation between acetaminophen use by expectant mothers and a heightened chance of their children receiving a diagnosis of autism spectrum disorder (ASD), ADHD, hyperactivity, or related conditions. These findings are supported by robust and consistent evidence.
The vulnerability of newborns to pharmaceuticals after the severance of the umbilical cord, as emphasized by visiting scholar Parker, aligns with ongoing product liability lawsuits against Tylenol's manufacturers. These lawsuits accuse the manufacturers of inadequately warning about the potential risks associated with prenatal acetaminophen exposure.
While autism spectrum disorder can have multiple causes, including genetic and environmental factors, the role of environmental influences, such as prenatal medication exposure, is a growing concern. Given the prevalence of ASD, affecting approximately 1 in every 50 to 60 children, these findings have led to numerous product liability lawsuits evaluating the scientific data on prenatal acetaminophen exposure.
Individuals affected by these potential risks are seeking to understand their eligibility for Tylenol-autism claim settlements, reflecting the significant impact this issue has on families and the wider public. Parents and healthcare providers must stay informed about the latest research findings to make well-informed decisions regarding medication use during pregnancy.
The alleged link between Tylenol and autism has had far-reaching implications for the pharmaceutical industry. Companies are reassessing their marketing and sales strategies while facing questions about their reputations. Ongoing legal proceedings and settlement negotiations underscore the seriousness of the issue and the public's demand for accountability.
In conclusion, the potential risks associated with acetaminophen use during pregnancy require a proactive approach to ensure the well-being of children. Parents and healthcare providers should seek reliable information and resources to navigate the complexities of medication use during pregnancy. The ongoing legal actions and scientific scrutiny serve as a reminder of the need for clear guidance and accountability within the pharmaceutical industry.




